Describe the Dual Nature of Light
Which scientists supported the idea that light is made of streams of particles. Its important postulates are.
The Wave Particle Duality Of Photons Nature Of Light Photon Terrace
Light is a wave or disturbance that travels through spaces in the air.

. The first theory defines light as particles and the second theory as waves. Particle Nature of Light or Plancks Quantum Theory. Each quanta has definite amount of energy which depends upon frequency of radiation.
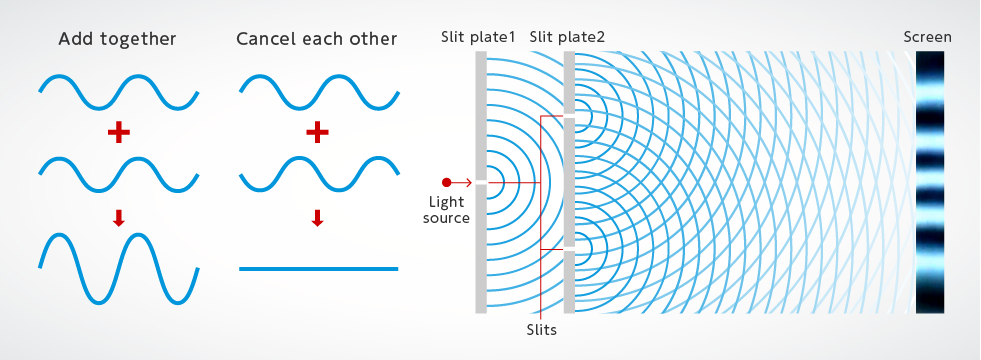
In 1801 Thomas Young shined light between two adjacent slits. Describe the color of light that passes through different colored filters such as red green and blue. Light is a substance carrying particles that flow from a light source.
A What does de Broglies equation indicate about the nature of matter. Electromagnetic fields perpendicularly oscillate to the direction of wave travel and are perpendicular to each other. The dual nature of light means that light can act as both a wave and a particle.
We call this twofold nature the particle-wave duality meaning that EM radiation has properties of both particles and waves. At first physicists were reluctant to accept the dual nature of light. But Einstein paved the way in 1905 by embracing wave-particle dualityWeve already discussed the photoelectric effect which led Einstein to.
Dual Nature of Light Theory. 2 The dual nature of light means light behaves as both wave and par. View the full answer Transcribed image text.
School University of South Florida. According to this theory small particles like electrons when in motion possess wave properties. There are some aspects of light that can be explained only if we think of light as being a wave reflection for example or light passing through a prism or grating to produce the rainbow of colors and other aspects of light that can be explained as if light was a particle.
In other experiments light behaves as a particle. Maxwells equation of electromagnetism and Hertz experiments on the generation and detection of electromagnetic waves in 1887 strongly established the wave nature of light. Sometimes it behaves like a wave which explains how light bends or diffracts around an object 3.
Quantum mechanics gave proof of the dual nature of light. The dual nature of light refers to the fact that light can act as both a wave and a particle. Light Wave Theory Most of the time light behaves as a wave and it is categorized as one of the electromagnetic waves because it is made of both electric and magnetic fields.
This experiment captured the dual nature of the photon by a special camera for the first time ever in the world and you can check it out on the video below. What is the speed of light in a vacuum. In physics there are two theories by which light can be defined.
Another theory says that light is a wave traveling with characteristics similar to those of water waves. Quantum mechanics considers the wave behavior of the electron while the Bohr model. Check all that apply.
When light waves are forced to travel through very small areas like the two tiny holes in Youngs experiment the interference pattern that is created can only result if light is a wave not a particle. Quantum of light is called a photon. Considers the particle nature of the electron.
Describe the evidence for the wave particle dual nature of light 1 Which of the. The transverse nature of light can be demonstrated through polarization. The relation is E hυ Where E is energy of photon h is Plancks.
This may seem contradictory since we ordinarily deal. The light waves interfered with each other and formed an alternating pattern of light and dark bands. Albert Einsteins photoelectric effect experiment proves that light can behave as a particle while Thomas Youngs double-slit experiment shows that it also behaves as a wave.
The question speaks to the so-called duality of light. The light bands are the constructive. Show more Science Physics This question was created from Module 7 DBAdocx.
We have long known that EM radiation is like a wave capable of interference and diffraction. The location of the particle is known in the Bohr model. Course Title CHEM 2045.
Compare and contrast the frequency. 2 This presented a new wave mechanical theory of matter. Therefore a dual nature of light theory was proposed up to now it is considered to be true.
1Sometimes it behaves like a particle called a photon which explains how light travels in straight lines 2. The dark band is the absence of light or when light waves cancel each other out while the lighter bands are where light waves amplify each other. Describe the evidence for the wave particle dual.
In the quantum mechanics. Like all electromagnetic waves light can travel through a vacuum. Einstein was able to prove that light can behave as a partide by showing that a beam of light could eject electrons from metal.
Thus the wave-particle duality is an important concept in quantum mechanics which describes that every particle or more specifically quantum entity may be expressed as either a particle or a wave. The dual nature of light means that in some experiments light behaves as a wave. Describe the dual nature of light.
Explain what color a person sees for various combinations of red green and blue light. Light is a transverse electromagnetic wave that can be seen by the typical human. This theory also fails because many but not all phenomena of light can be explained using this theory.
Dual Nature of Electron 1 In 1924 the French physicist Louis de Broglie suggested that if light has electron behaves both as a material particle and as a wave. Quantum theory was given by Max Planck in 1900. The wave nature of light was first illustrated through experiments on diffraction and interference.
The nature of the photon Based on Einsteins light quantum hypothesis the duality of the photon was confirmed quantum-mechanical experiments and examination. We now see that light can also be modeled as particlesmassless photons of discrete energy and momentum. Discuss the dual nature of light Electromagnetic or EM Waves.
Pages 12 Ratings 100 4 4 out of 4 people found this document helpful. As long as electrons remain in its orbit it emits no radiation. After all many of us humans like to have one right answer.
Describe what is referred to as the dual nature of light and provide the evidences that prove the dual nature of light. Scientists accept the evidence that.

Comments
Post a Comment